By Aditya Suroshe1*, Ganga Sahay Meena2* and Anil V1
1 Research Scholar, M. Tech. (Dairy Technology)
2 Senior Scientist,
Dairy Technology division ICAR-national Dairy Research Institute Karnal, Haryana, India, 132001
*Corresponding author: [email protected] (office: +91 1842259485)
Abstract
The dairy industry has seen tremendous expansion and development in recent years. This necessitates a greater focus on quality control throughout the manufacturing process. The need for food safety, as well as the recent growth in dairy product consumption globally, have raised interest in future research in these areas. Dairy products are vulnerable to adulteration due to their high demand. Infrared spectroscopic techniques have been utilized not only to verify the authenticity of dairy products but also to define their quality. IR spectroscopy may also be utilized to help with problems regarding cheese ripening, composition, and contamination. This article focuses on some of the research that uses infrared spectroscopy to determine the intrinsic quality of dairy products.
Introduction
Milk and milk products are well-known sources of nutrients essential to human health. Their consumption has recently increased dramatically. As a result, considerable effort has gone into developing high-quality, innovative dairy products that are more appealing to customers while also meeting functional requirements for human health and nutrition. Due to the discovery of many serious contamination instances over the last decade, the authenticity of milk products has also become a critical problem. Therefore, assessing food quality and authenticity is a critical issue for regulatory bodies, food processors, and end users. So, it is essential to assure the authenticity of the component by regularly checking its quality in the food industry through the adoption of sophisticated and precise new methodologies.
Infrared spectroscopy is quickly becoming the most extensively used tool for determining food authenticity and adulteration/contamination, and it is a critical technology in the dairy sector for raw material analysis, process management, and final product composition [7]. Whey, WPCs, and cheeses have all been successfully quality-tested utilizing technologies such as mid-infrared (MIR) and near-infrared (NIR) techniques[1,2,4,13,20].
Basically, optical spectroscopic techniques evaluate the quality of the food by generating a unique “fingerprint”.When a dairy product with a certain chemical composition is exposed to light, it produces a distinct spectrum as a result of the absorption of electromagnetic radiation by various chemical ingredients of the food[21].
As infrared spectroscopy is a non-destructive process, it is used widely in laboratories and at industrial scale (in-line and on-line monitoring). Although applications of in-line monitoring are limited but are helpful in process control by monitoring crucial attributes (e.g. recording real-time production data), which helps in rapid and economic operation. On the other hand, on-line applications mainly focus on final products’ labelling specifications by using reference methods for developing prediction models[7]. Both applications can improve profitability, increase efficiency, and ultimately enhances the quality of the final product. Thus, the infrared spectroscopy technique can be a key alternative to basic conventional techniques that are time-consuming, labour-intensive, and based on wet chemistry for determining the amount of compound present in test material [8].
Near-infrared (NIR) spectroscopy is based on molecular overtones and combination vibrations and operates in the near-infrared region of the electromagnetic spectrum (from 750 to 2500 nm or 14290 to 4000 cm-1) [12]. Spectra can be captured in transmission, reflection, or interactance modes, revealing significant chemical and physical information. Overtones and vibrational combinations, in particular C-H, O-H, N-H, and C=O, enable measurements of various structures [16].
Mid-infrared (MIR) spectroscopy (from 2500 to 25000 nm or 4000 to 400 cm-1) analyses the basic vibrational and rotational stretching modes of molecules, which represent the sample’s chemical makeup [12]. Because the approach monitors fundamental vibrations rather than overtones and combination bands as in the NIR range, it provides more chemical information about the scanned material. The primary bands in the MIR spectra are classified into two unique regions: the “functional group region” (4000 to 1500 cm-1) and the “fingerprint region” (1500 to 500 cm-1) [12]. Though, the functional group region, which is often complicated, provides the majority of critical information needed to interpret MIR spectra. As each molecule creates a separate and unique absorption pattern in this area, it contains a lot of structural rather than functional group information, as well as numerous overlapping bands [12].
Recent Applications of Infrared Spectroscopy for Assessment of Dairy Products’ Quality
As MIR and NIR are complementary spectral methods, they have advantages and limitations. MIR is the technique of choice for composition and quality controls during regular liquid milk testing across the world. It enables quick, non-destructive measurement of milk chemical characteristics while avoiding time-consuming, costly, and labour-intensive reference procedures [10]. MIR is useful for both qualitative and quantitative identification, with well-defined bands for organic functional groups, fat, protein, and lactose found in milk [5]. In comparison to NIR equipment, mid-infrared devices require a smaller sample amount for milk analysis.Though, its primary drawback is the existence of a large band of water absorption, as milk contains around 87% water [5]. Another downside of MIR is the necessity for sample preparation (in the absence of ATR), and there is experimental complexity in the analysis of milk. In comparison to the MIR, the NIR offers several benefits, including simpler and less expensive transmission equipment with glass lenses and a less expensive light source. The NIR is economically favourable for dairy tests since the samples are examined daily. Water over to NES causes weak absorption, allowing for the examination of high-moisture products. The NIR produces spectral data with exceptionally high signal-to-noise ratios [5]. The benefits of NIR spectroscopy include the ability to quantify the quantity of milk ingredients quickly and simultaneously, as well as the opportunity for online analysis. Spectral information is related to overtones and vibrational combinations of distinctive bonds, such as C-H, N-H, O-H, and S-H, which are found in all organic compounds [10]. The drawback of NIR is that the spectra are less prominent, resulting in more modest signal variations. NIR has a limited ability whileestimating heterogeneous samples[16]. In general, it is unable to distinguish components with concentrations less than 1% [5]. Furthermore, the reference techniques’ low accuracy and subjectivity are limitations for their uses. As a result, accurate calibrations for improved sampling techniques and reference systems are required.
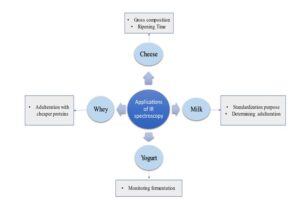
Various applications of IR Spectroscopy in dairy industry has been depicted in Figure 1 and criefly discussed here under.
Milk
Dairy products, such as milk and cheeseare high in nutritional content and consumed globally. Owing to their high market value, some suppliers frequently add adulterants to milk, such as salt, starch, melamine, citrate, sucrose, urea, water, and other components, in order to increase profits. Adulterated milk will not only put people in danger of disease but will also result in significant losses for the dairy sector, because milk quality is so vital in the manufacturing of all sorts of dairy products[18].
Effective use of NIR spectroscopy has been doneto distinguish raw cow milk from that contaminated with various pseudo proteins, thickeners [21], and melamine, a nitrogen-rich chemical toxic to human health [6].
Mid-infrared spectroscopy is commonly utilized for raw milk by processors for standardization, by the dairy sector for milk payment, and by farmers, technicians, and businesses that employ a variety of milk characteristics. MIR spectroscopy was also been used to determine the Solid Non-Fat (SNF) content of raw milk in a quick and non-destructive manner [3].The use of Mid-infrared spectroscopy and multivariate supervised classification to identify five adulterants (formaldehyde, sucrose, water, starch, and sodium citrate) in raw cow milk was carried out by Botelho et al. [4]. It was discovered that for each type of adulterant, a specific portion of the spectrum differs from the original milk spectrum. In the case of formaldehyde adulteration, a distinct peak of approximately 1000 cm-1 could be observed. The presence of numerous peaks in the fingerprint region (near 1200 – 1000 cm-1) was shown to be linked with differentiation in sucrose
Establishment of spectroscopic patterns of antibiotics isolated and in the presence of milk was done by Teixeira et al. [19], which adds another tool for the rapid and precise identification of trace quantities of these chemicals. They employed vibrational spectroscopy as well as theoretical calculations based on Density Functional Theory in bovine milk to accomplish this.
Whey and its Products
Due to the increasing demand for goods with healthy and high nutritional content, the dairy sector has recognized the importance of whey proteins. Whey accounts for around 85-90 percent of the amount of milk used in cheese making and maintains approximately 55% of the milk’s nutrients [17]. Despite its high concentration of beneficial nutrients, whey was previously seen as a waste product and an environmental contaminant with little economic application. However, this by-product of cheese production is increasingly finding new technological and nutritional applications. Whey proteins may be included in a variety of food items to preserve their functional and nutritional value, and they can also be utilized as nutritional supplements in the sports nutrition industry [17].Whey Protein Concentrate (WPC), Whey Protein Isolate (WPI), and Whey Protein Hydrolysates (WPH) are the most common examples in this category. They have a protein content of 65-80% and above 90% on a dry basis, respectively, and are high in bioactive peptides, essential amino acids, and antioxidants [14]. As WPC and WPI have significant added value, they have become targets for adulteration with cheaper components such as amino acid derivatives, carbohydrates, and thermogenic compounds [15].
Wang et al. [20] combined the spectral signature gathered from portable mid-infrared spectrometers with pattern recognition analysis to establish a simple and quick approach for differentiating whey protein classes (WPC, WPI, and WPH) used in beverage production. The findings revealed that the principal bands centred at 1640 and 1580 cm-1 were responsible for the separation and related to variations in proteins’ amide I and amide II vibrations, respectively. In general, Attenuated Total Reflectance (ATR) spectroscopy paired with pattern recognition chemometrics can differentiate and authenticate commercial whey protein components in the infrared region.
O’Loughlin et al. [13] studied the thermal denaturation of whey protein solutions by physically analysing the solutions and using infrared spectroscopy to characterize the changes that occur in whey protein dispersions upon heat treatment. According to them, Infrared (IR) spectroscopy is faster and less expensive method to determine protein structure (2° and 3°) as compared with other methods. Their findings showed that in homogenous protein systems, MIR spectroscopy is a valuable technique for determining structural changes related to physical attributes.
The possible use of Fourier-Transform Infrared Attenuated Total Reflectance (FTIR-ATR) to identify Whey Protein Concentrate (WPC) adulteration by milk whey powder (MWP) was established by Andrade et al. [1]. It was discovered that the region from 1700 to 1500 cm-1 had the largest peaks, features of Amide I (ν C=O, ν C-N) in 1640 cm-1 and Amide II (δ N-H, ν C-N) in 1550 cm-1, with substantial differences across samples. Principal Component Analysis (PCA) and Partial Least Squares (PLS) regressions were used to model and forecast the protein content as well as the amount in grams of WPC and MWP in the samples.The authors concluded that the combination of FTIR-ATR spectroscopy and multivariate techniques has great potential for detecting adulteration in nutritional supplements and high accuracy in predicting the protein content and mass of WPC and MWP added.
Cheese
Cheese is the most prevalent processed dairy product in the world, and it supplies critical nutrients to consumers. As a result, quality monitoring of cheeses is critical. Several researchers have used infrared spectroscopy to assess cheese quality, although traditional approaches are time-consuming and costly. The majority ofworks are about the ripening of cheese, composition, and validity or authentication.
Andrade et al. [2] used FTIR/ ATR spectroscopy to analyze the ripening changes over time of cheeses manufactured from ewes’ milk (Pecorino, ewes’ ripe, and Gouda. The researchers were able to identify proteolysis and lipolysis, two fundamental events that characterize cheese ripening. Overall, there was a declining trend in the absorbance intensity of the amide group peaks (1700 to 1500 cm-1), which is connected to peptide bond breaking. The lipidic area (3000 to 2800 cm-1 and 1765 to 1730 cm-1) showed a similar trend. During the first trimester of the ripening process, proteolysis was rapid, and the lipids were gradually transformed into smaller species with time. The physicochemical changes of the cheeses were evaluated using hierarchical cluster analysis and principal component analysis.
Leite et al. [9] employed MIR spectroscopy to detect butter cheeses contaminated with soybean oil at amounts ranging from 0% to 100% substitution [9]. The primary bands seen in the spectra were associated with the presence of water, lipids, and proteins, all of which are found in the “functional group” range (4000 to 1500 cm-1) [11]. Overall, adulteration caused a rise in absorbance values of up to 70% in the lipid-related bands. The band near 3007 cm-1, which represents stretches of groups -C=CH (cis-) of double bonds in unsaturated fatty acids, was the only one with a consistent increase in absorbance values due to the increase in soybean oil content in the cheeses, even in those with 80%, 90%, and 100% adulteration. The band shift was also found in this peak and was utilized to describe the adulterations.As a result, it was discovered that the amount and content of unsaturated fatty acids (which are abundant in soybean oil but lacking in butter oil) alter the position and strength of this band, indicating fraud.
Conclusion & Future Scope
The assessment of food quality and authenticity necessitates thorough food monitoring using effective analytical techniques with precise results. It can be established that various IR spectroscopy techniques (NIR and MIR)havethe potential for analysing food safety qualities, identifying adulterants, and conducting dairy product characterization and categorization. Furthermore, the utility of infrared spectroscopy is enhanced by its application in chemometrics. It makes MIR and NIR systems more appropriate and effective for analysing a wide range of items.Additional human upskilling is necessary at the industrial level for the development, assessment, and maintenance of strong calibration models to assure the reliability of IR equipment.
Future work on the connection and communication between the IR measuring system and the control system is required to close the loop of ‘process, monitoring and control’.
References
- Andrade, J., Pereira, C. G., de Almeida Junior, J. C., Viana, C. C. R., de Oliveira Neves, L. N., da Silva, P. H. F. & dos Anjos, V. D. C. (2019). FTIR-ATR determination of protein content to evaluate whey protein concentrate adulteration. Lwt, 99, 166-172.
- Andrade, J., Pereira, C. G., Ranquine, T., Azarias, C. A., Bell, M. J. V., & de Carvalho dos Anjos, V. (2018). Long-term ripening evaluation of ewes’ cheeses by fourier-transformed infrared spectroscopy under real industrial conditions. Journal of Spectroscopy, 2018.
- Bassbasi, M., Platikanov, S., Tauler, R., & Oussama, A. (2014). FTIR-ATR determination of solid non fat (SNF) in raw milk using PLS and SVM chemometric methods. Food chemistry, 146, 250-254.
- Botelho, B. G., Reis, N., Oliveira, L. S., & Sena, M. M. (2015). Development and analytical validation of a screening method for simultaneous detection of five adulterants in raw milk using mid-infrared spectroscopy and PLS-DA. Food chemistry, 181, 31-37.
- Brown, G. (2013). Quality Control using NIR/MIR Spectroscopy: A Rotten Apple Could Turn Your Product into a Lemon. IICA Tech Night Presentation, 9.
- Chen, H., Tan, C., Lin, Z., & Wu, T. (2017). Detection of melamine adulteration in milk by near-infrared spectroscopy and one-class partial least squares. Spectrochimica Acta Part A: Molecular and Biomolecular Spectroscopy, 173, 832-836.
- De Marchi, M., Penasa, M., Zidi, A., & Manuelian, C. L. (2018). Invited review: Use of infrared technologies for the assessment of dairy products-Applications and perspectives. Journal of dairy science, 101(12), 10589-10604.
- Karoui, R., & De Baerdemaeker, J. (2007). A review of the analytical methods coupled with chemometric tools for the determination of the quality and identity of dairy products. Food chemistry, 102(3), 621-640.
- Leite, A. I. N., Pereira, C. G., Andrade, J., Vicentini, N. M., Bell, M. J. V., & Anjos, V. (2019). FTIR-ATR spectroscopy as a tool for the rapid detection of adulterations in butter cheeses. Lwt, 109, 63-69.
- Liu, N., Parra, H. A., Pustjens, A., Hettinga, K., Mongondry, P., & Van Ruth, S. M. (2018). Evaluation of portable near-infrared spectroscopy for organic milk authentication. Talanta, 184, 128-135.
- Lohumi, S., Lee, S., Lee, H., & Cho, B. K. (2015). A review of vibrational spectroscopic techniques for the detection of food authenticity and adulteration. Trends in Food Science & Technology, 46(1), 85-98.
- Nunes, C. A. (2014). Vibrational spectroscopy and chemometrics to assess authenticity, adulteration and intrinsic quality parameters of edible oils and fats. Food Research International, 60, 255-261.
- O’Loughlin, I. B., Kelly, P. M., Murray, B. A., FitzGerald, R. J., & Brodkorb, A. (2015). Concentrated whey protein ingredients: A Fourier transformed infrared spectroscopy investigation of thermally induced denaturation. International Journal of Dairy Technology, 68(3), 349-356.
- Patel, S. (2015). Functional food relevance of whey protein: A review of recent findings and scopes ahead. Journal of Functional Foods, 19, 308-319.
- Pereira, C. G., Andrade, J., Ranquine, T., de Moura, I. N., da Rocha, R. A., Furtado, M. A. M. & Anjos, V. (2018). Characterization and detection of adulterated whey protein supplements using stationary and time-resolved fluorescence spectroscopy. Lwt, 97, 180-186.
- Qu, J. H., Liu, D., Cheng, J. H., Sun, D. W., Ma, J., Pu, H., & Zeng, X. A. (2015). Applications of near-infrared spectroscopy in food safety evaluation and control: a review of recent research advances. Critical Reviews in Food Science and Nutrition, 55(13), 1939-1954.
- Sinha, R., Radha, C., Prakash, J., & Kaul, P. (2007). Whey protein hydrolysate: Functional properties, nutritional quality and utilization in beverage formulation. Food Chemistry, 101(4), 1484-1491.
- Summer, A., Franceschi, P., Bollini, A., Formaggioni, P., Tosi, F., & Mariani, P. (2003). Seasonal variations of milk characteristics and cheesemaking losses in the manufacture of Parmigian -Reggiano cheese. Veterinary research communications, 27, 663.
- Teixeira, R. C., Luiz, L. C., Junqueira, G. M. A., Bell, M. J. V., & Anjos, V. C. (2020). Detection of antibiotic residues in Cow’s milk: A theoretical and experimental vibrational study. Journal of Molecular Structure, 1215, 128221.
- Wang, T., Tan, S. Y., Mutilangi, W., Aykas, D. P., & Rodriguez‐Saona, L. E. (2015). Authentication of Whey Protein Powders by Portable Mid‐Infrared Spectrometers Combined with Pattern Recognition Analysis. Journal of food science, 80(10), C2111-C2116.
- Zhang, L. G., Zhang, X., Ni, L. J., Xue, Z. B., Gu, X., & Huang, S. X. (2014). Rapid identification of adulterated cow milk by non-linear pattern recognition methods based on near infrared spectroscopy. Food chemistry, 145, 342-348.
Figure 1. Applications of IR Spectroscopy in dairy industry